Projects and Case Studies
Every project we make is the result of a shared path, made of listening, analysis and tailor-made solutions. We collaborate with complex realities in clinical, scientific and managerial fields, transforming the needs into robust, scalable and secure software. Whether it is a clinical trial management system, a data collection platform or mission-critical software infrastructure, we always focus on technological reliability and long-term vision.

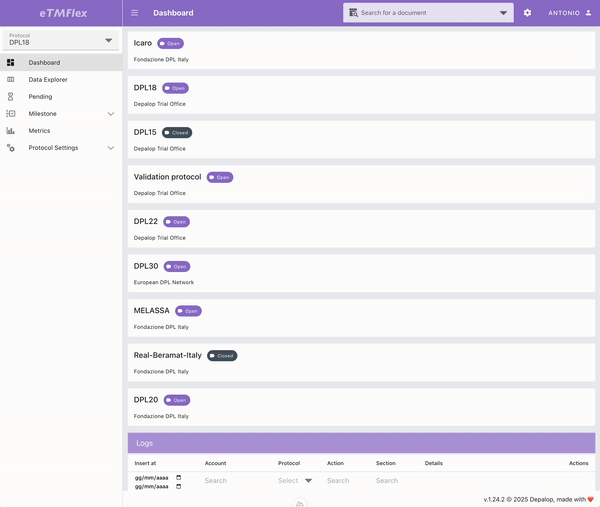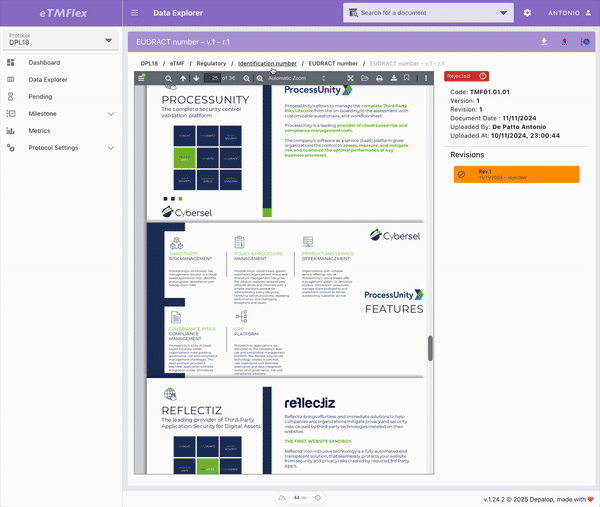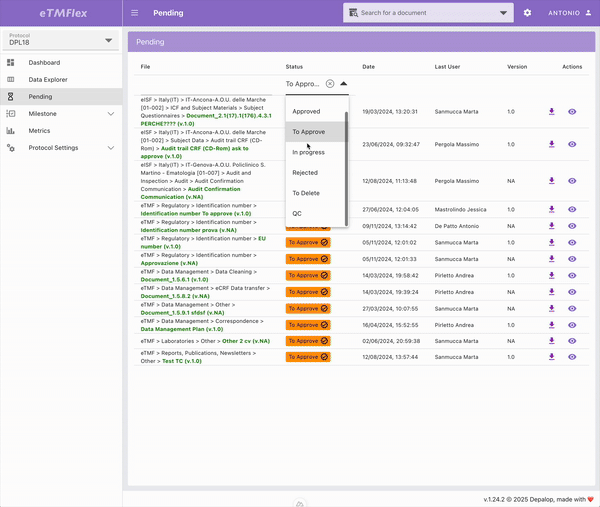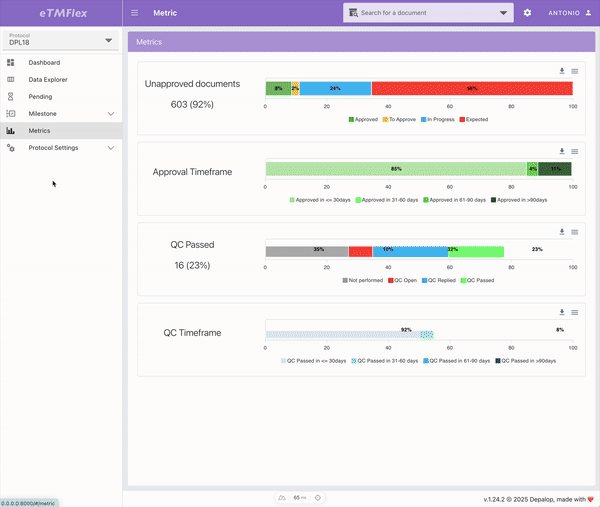
eTMFlex
eTMFlex is an advanced eTMF (electronic Trial Master File) system for clinical trial document management, designed to provide maximum flexibility and control over the configuration and ongoing update of the document structure. At the core of the platform is a dynamic Data Dictionary, enabling secure and traceable creation and synchronization of the document tree via Excel files, while preserving data integrity. eTMFlex introduces innovations such as symlink support, readable document codes via customizable templates, and a granular system of user-defined milestones. It’s a powerful and highly configurable platform in managing clinical trial documentation.

Unlock REDCap’s Full Potential
REDCap is a powerful tool for the collection and management of clinical data, but to fully exploit its potential, we need technical skills, adequate infrastructure and customizations that meet the real needs of your research team.
Our service was created to simplify the management of REDCap, offering you a complete, secure and personalized solution, without having to take care of the technical part.
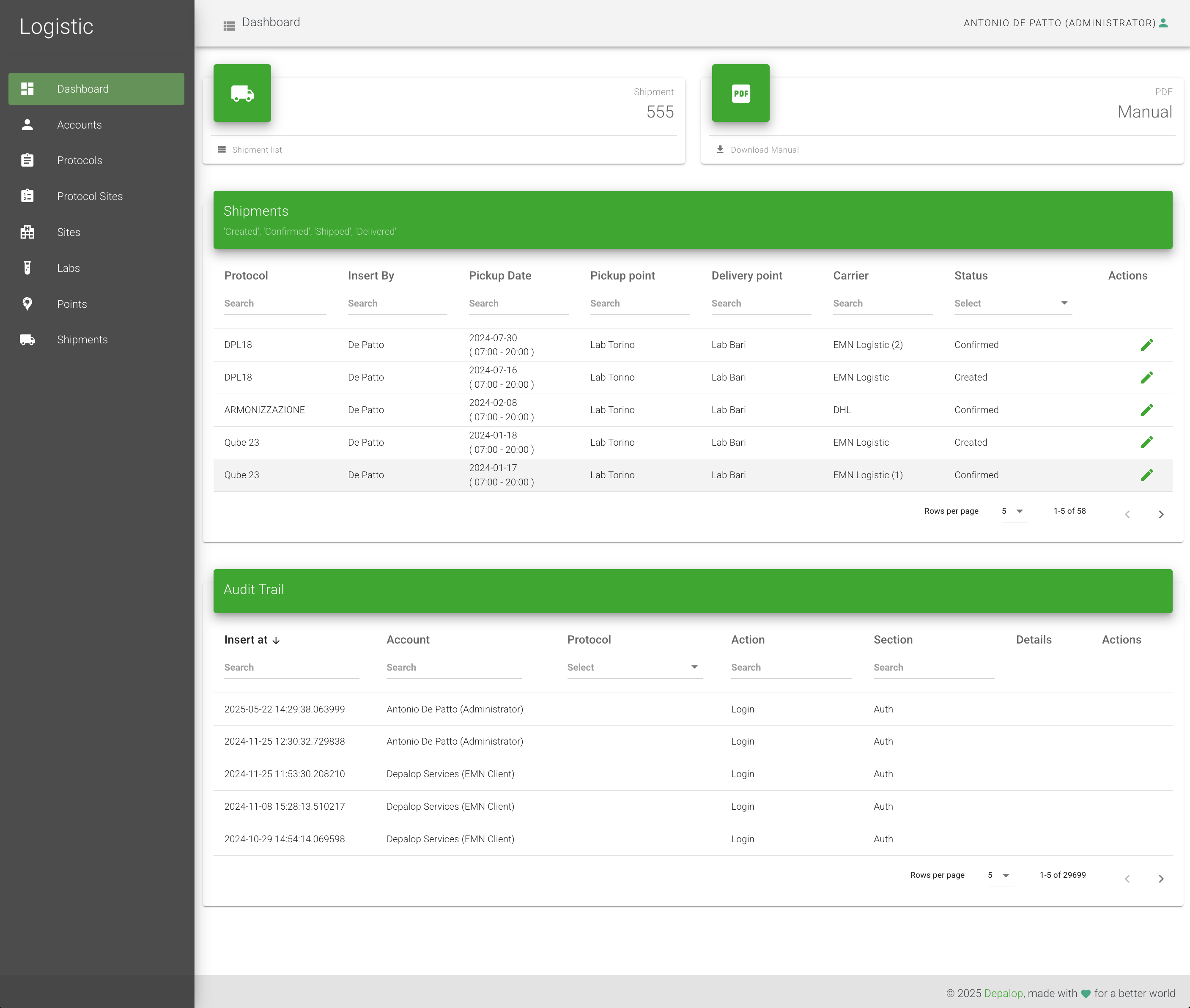
Logistics
Logistic is a web-based platform for clinical logistics management, designed to ensure the traceability and timely delivery of biological samples in clinical trials. It enables sponsors, CROs, and laboratories to efficiently coordinate shipments between clinical sites and testing laboratories, ensuring regulatory compliance and sample integrity. Through centralized monitoring, automated notifications, and advanced tracking tools, Logistic enhances stakeholder collaboration and reduces operational risk, making it a strategic solution for managing complex clinical studies.

SafetyDatabase
SafetyDatabase is a software system designed for the complete management of serious adverse events (SAE) in clinical trials, in accordance with European CT-3 and GVP (Good Pharmacovigilance Practices). The system allows clinical trial sponsors to comply with regulatory obligations and best practices regarding pharmacovigilance, ensuring traceability, quality and integrity of data. SafetyDatabase complies with FDA 21 CFR Part 11 regulations for electronic systems and can be integrated with eCRF such as REDCap, custom systems and Excel files.

eCDS
The eCDS (Electronic Clinical Drug Supply) project is designed to simplify and digitize the management of experimental drugs (IMP – Investigational Medicinal Products) within clinical studies. The system addresses one of the most widespread criticalities in pharmacovigilance and clinical logistics: manual management, often based on emails and Excel sheets, requests, distributions, confirmations and disposals of drugs. Through a centralized platform, eCDS allows precise batch monitoring, waste reduction and constant control over the entire drug life cycle, ensuring greater efficiency, traceability and regulatory compliance.

SpaceMuseum
SpaceMuseum is an innovative application that transforms your smartphone into a new generation interactive audioguide. Through an intelligent system based on the analysis of visitor movements, the app is able to detect in real time the work to which attention is concentrated, automatically activating dedicated audio, video and text content.

StoreFinder
The StoreFinder project stems from the need to overcome the inefficiencies of the traditional anaographic databases related to the stores, often obsolete and incomplete. In a context in which business activities open, close or change use destination quickly, relying exclusively on these sources can compromise the quality of market analysis.
